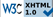Directed Evolution of proteins for Biofuel cell applications (Strasbourg).
Digital microfluidic systems for the directed evolution of catalysts for biofuel cells
Scientists involved: Thomas Beneyton (see Project), Chaoucki Ben Salem, Yousr Skhiri (see Project)
Scientists involved previously: Dr. Abdeslam El Harrak, Dr. Felix Kleinschmidt, Dr. Linas Mazutis
Our final goal is to build a functional bio-fuel cell using enzymes as redox catalysts at the anode and cathode. To achieve this goal the key points are a high catalytic activity of these enzymes at high ethanol concentrations, broad pH and temperature variations and their stability at the interface with the electrodes. These are conditions under which the natural enzyme will never evolve in vivo. Hence, we are aiming to apply in vitro protein evolution methods in combination with digital microfluidics to create better catalysts for the "green energy" production.
The expenses concerning "green energy" production might be overcome by availability of better bio-catalysts. In the most common way biofuel, in form of ethanol, is obtained from sugars stored within the plants such as corns, sugarcane.
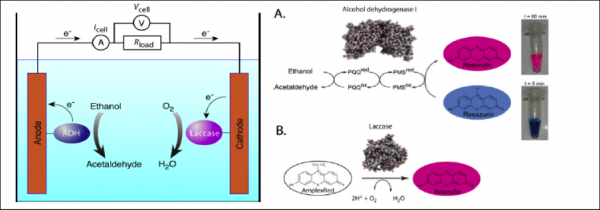
Schematic configuration of a biofuel cell based on redox enzymes and the fluorogenic tests for measuring the activity of the biocatalysts in droplet: (On the left) ethanol is oxidized to acetaldehyde by alcohol dehydrogenase (blue ellipse). During oxidation, electrons from ethanol are transferred onto the anode via PQQ cofactor, while protons are released into the media. At the cathode, electrons are transferred to immobilized laccase (purple ellipse), which catalyzes oxygen reduction to water in the presence of copper ions. (On the right) panel A: the fluorogenic assay for the PQQ-ADH: in the presence of ethanol, the Pyrroloquinoline quinine (PQQ) cofactor and the PMS (Phenazine MethoSulfate: electron mediator), resazurin is converted to resorufin. (On the right) p anel B: the fluorogenic assay for the laccase: in the presence of oxygen, AmplexRed is converted to resorufin. Taken from Skhiri et al., Proceedings MicroTas 2010.
Fundings
ADEME, Région Alsace, Fondation d'entreprise EADS, Université de Strasbourg, CNRS.
Collaborators
Prof. P. Hellwig (see http://www-chimie.u-strasbg.fr/~lsveb/ ).
Prof. Dr. Wolfgang Schuhmann (http://www.ruhr-uni-bochum.de/elan/ )
Dr J.-C. Baret (http://dmi.ds.mpg.de/ )
Publications
CotA laccase: high-throughput manipulation and analysis of recombinant enzyme libraries expressed in E. coli using droplet-based microfluidics. Beneyton, T., Coldren, F., Baret, J-C., Griffiths, A.D. and Taly, V.* Analyst (2014), 10.1039/C4AN00228H (Invited article for the themed issue on “Probe and Chip Approaches to Cell Analysis”).
Membraneless Glucose/O2 microfluidic biofuel cells using covalently bound enzymes. Beneyton, T., I Putu Mahendra Wijaya, C. Ben Salem, C., Griffiths, A.D.* and Taly, V.* Chemical communications (2013), 49(11): 1094-1096. Journal Link.
The thermophilic CotA laccase from Bacillus subtilis: Bioelectrocatalytic evaluation of O2 reduction in the direct and mediated electron transfer regime. Beneyton, T., Beyl, Y., Guschin, D., Griffiths, A.D., Taly, V. and Schuhmann, W. Electroanalysis (2011), 22: DOI: 10.1002/elan.201100054. Link
Immobilisation of CotA, an extremophilic laccase from Bacillus subtilis, on glassy carbon electrodes for biofuel cell applications. Beneyton, T., El Harrak, A., Griffiths, A.D. and Taly, V. Electrochemistry Communications (2011), 13(1): 24-27. Link
Integrated microfluidic platform for directed evolution of laccase for Biofuel cell applications. Skhiri, Y., Beneyton, T., El Harrak, A., Griffiths, A.D. and Taly, V. In: Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS), 2010, p. 980-982. ISBN: 978-0-9798064-3-8.
Multi-step microfluidic droplet processing: kinetic analysis of an in vitro translated enzyme. Mazutis L., Baret, J. C., Treacy, P., Skhiri, Y., Fallah-Araghi, A., Ryckelynck, M., Taly, V. and Griffiths, A.D. Lab on a Chip (2009), 9: 2902-8. Pubmed